Antibiotics have been a wonderful invention in the evolution of modern medical treatments. The mortality and the morbidity due to different infectious diseases have remarkably gone down over the last many decades. However pathogens have emerged as even more smarter than mankind developing different complex resistance mechanisms to survive the assault of antibiotics thereby rendering treatment of infectious diseases a difficult challenge. Patients with Diabetic Ketoacidosis, Chronic Renal failure, Implants & Prosthesis often land up with sepsis with multi drug resistant organisms ,treatment of which pose a serious challenge to the treating clinicians. To combat this crisis, antibiotic stewardship practice has become the need of the hour which emphasize the rational & judicious use of all available antibiotics. The regulatory authority should be more vigilant in preventing the over the counter dispensing of antibiotics.
Having said that, all the medical fraternities and academic forums should take initiatives to implement antibiotic stewardship practices in day to day clinical practices. Let us have a quick brush up of the antibiotic resistance mechanisms.
ESBLs :
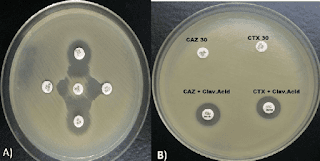
Due to an increase in community-acquired infections, ESBL-E has become more frequently detected in bacterial cultures.The majority of penicillins, cephalosporins, and aztreonam are rendered inactive by ESBL enzymes. Carbapenems continue to generally be effective against EBSL-E. ESBLs do not render non-lactam agents inactive (e.g., ciprofloxacin, trimethoprim-sulfamethoxazole, gentamicin). However, organisms with ESBL genes frequently have extra genes or gene mutations that cause resistance to a variety of antibiotics.
Although ESBL genes can be found in any Gram-negative organism, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis have the highest rates of occurrence. The most widespread CTX-M enzymes are CTX-M-15. Other ESBLs with distinctive hydrolyzing properties exist in addition to CTX-M enzymes, such as variants of narrow-spectrum TEM and SHV -lactamases with amino acid substitutions, though they have undergone less thorough clinical testing. Most clinical microbiology laboratories don't conduct routine EBSL testing. Although this threshold has limitations with specificity because organisms that are not susceptible to ceftriaxone for reasons other than ESBL production may be mistakenly assumed to be ESBL-producers, non-susceptibility to ceftriaxone (i.e., ceftriaxone minimum inhibitory concentrations [MICs] 2 mcg/mL) is frequently used as a proxy for ESBL production.
CRE :
Members of the Enterobacterales order who are resistant to at least one carbapenem antibiotic or who produce a carbapenemase enzyme are known as CRE, according to the CDC .Resistance to at least one carbapenem other than imipenem is necessary for bacteria that are inherently resistant to imipenem (such as Proteus spp., Morganella spp., and Providencia spp.). The pathogens that cause CRE can be roughly divided into those that produce carbapenemase and those that do not into groups with different potential mechanisms of resistance. The amplification of non-carbapenemase -lactamase genes (other than carbapenemase genes) with concurrent outer membrane porin disruption may cause CRE that do not produce carbapenemase. 35% to 59% of CRE cases in the US are caused by isolates that produce carbapenemase.
K. pneumoniae carbapenemases (KPCs), which can be produced by any Enterobacterales, are the most prevalent carbapenemases. The Verona integron-encoded metallo-lactamases (VIMs), imipenem-hydrolyzing metallo-lactamases (IMPs), and oxacillinases (such as OXA-48-like) are additional notable carbapenemases that have been discovered . Making treatment decisions requires having knowledge of whether a CRE clinical isolate produces carbapenemase and, if so, what kind of carbapenemase is produced.
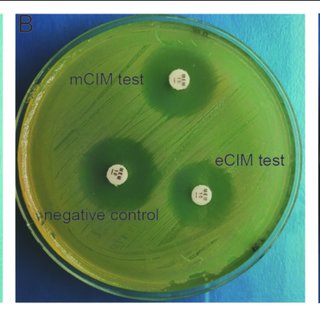
CRE that produce carbapenemase and those that do not can be distinguished by phenotypic tests like the modified carbapenem inactivation method and the Carba NP test. Molecular analysis can pinpoint particular carbapenemase families (e.g., differentiating a KPC from an OXA-48-like carbapenemase). A small number of clinical microbiology laboratories perform carbapenemase phenotypic and/or genotypic testing, but most of the international bodies strongly encourage all clinical microbiology laboratories to pursue carbapenemase testing in order to guide the best possible treatment choices. The following treatment suggestions for CRE infections presuppose that preferred and alternative antibiotics have shown in vitro activity.
MDR/DTR Pseudomonus aeruginosa:
Penicillins, cephalosporins, fluoroquinolones, aminoglycosides, and carbapenems are among the antibiotic classes for which P. aeruginosa susceptibility is typically anticipated. MDR P. aeruginosa is defined as P. aeruginosa not susceptible to at least one antibiotic in at least three antibiotic classes . The idea of "difficult-to-treat" resistance was put forth in 2018. DTR is defined as P. aeruginosa that does not exhibit sensitivity to any of the following drugs: piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin, and levofloxacin.
Multidrug-resistant P. aeruginosa, also known as DTR-P. aeruginosa, typically arises from the interaction of several complex resistance mechanisms, such as reduced expression of outer membrane porins (OprD), increased production of AmpC enzymes, increased activity of efflux pumps, and mutations in targets of the penicillin-binding protein . Carbapenemase production is a rare cause of carbapenem resistance in P. aeruginosa, but it has been found in up to 20% of carbapenem-resistant P. aeruginosa worldwide.
Table . Suggested dosing of antibiotics for the treatment of infections caused
by antimicrobial- resistant organisms
Reference : Infectious Disease Society of America (IDSA)
|
Agent |
Adult Dosage (assuming
normal renal and liver function ) |
Target Organisms |
|
Amikacin |
Cystitis: 15 mg/kg/dose IV once All other infections: 20 mg/kg/dose IV x 1 dose,
subsequent doses and dosing
interval based on pharmacokinetic evaluation |
ESBL-E, AmpC-E, CRE, DTR- P. aeruginosa |
|
Ampicillin-sulbactam |
9 g IV q8h
over 4 hours OR 27 g IV q24h
as a continuous infusion For
mild infections caused by CRAB isolates susceptible to ampicillin-sulbactam, it is reasonable to administer 3g IV q4h – particularly if intolerance or toxicities preclude the use of higher dosages. |
CRAB |
|
Cefepime |
Cystitis: 1 g IV q8h All other infections: 2
g IV q8h, infused over 3 hours |
AmpC-E |
|
Cefiderocol |
2 g IV q8h, infused over 3 hours |
CRE, DTR-P. aeruginosa, CRAB, S. maltophilia |
|
Ceftazidime- avibactam |
2.5 g IV q8h, infused over 3 hours |
CRE, DTR-P. aeruginosa |
|
Ceftazidime- avibactam and aztreonam |
Ceftazidime-avibactam: 2.5 g IV q8h,
infused over 3 hours PLUS Aztreonam: 2 g IV q8h, infused over 3 hours, administered at the
same time as ceftazidime-avibactam, if possible |
Metallo-β-lactamase- producing CRE, S. maltophilia |
|
Ceftolozane- tazobactam |
Cystitis: 1.5 g IV q8h, infused
over 1 hour All other infections: 3 g IV q8h,
infused over 3 hours |
DTR-P. aeruginosa |
|
Ciprofloxacin |
ESBL-E or AmpC infections: 400 mg IV q8h-q12h OR 500 – 750 mg PO
q12h |
ESBL-E, AmpC-E |
|
Colistin |
Refer to international consensus guidelines on
polymyxins |
CRE
cystitis, DTR-P. aeruginosa cystitis, CRAB
cystitis |
|
Eravacycline |
1 mg/kg/dose IV q12h |
CRE, CRAB |
|
Ertapenem |
1 g IV q24h,
infused over 30 minutes |
ESBL-E, AmpC-E |
|
Fosfomycin |
Cystitis: 3 g PO x 1 dose |
ESBL-E. coli cystitis |
|
Agent |
Adult Dosage (assuming
normal renal and liver function ) |
Target Organisms |
|
Gentamicin |
Cystitis: 5 mg/kg/dose IV once All other infections: 7 mg/kg/dose IV x 1 dose, subsequent doses
and dosing interval based on pharmacokinetic
evaluation |
ESBL-E, AmpC-E, CRE, DTR-P. aeruginosa |
|
Imipenem-cilastatin |
Cystitis (standard infusion): 500 mg IV q6h, infused over 30
minutes All other
ESBL-E or AmpC-E
infections: 500 mg IV q6h, infused over 30 minutes All other CRE and CRAB
infections: 500 mg IV q6h, infused over
3 hours |
ESBL-E, AmpC-E, CRE, CRAB |
|
Imipenem-cilastatin- relebactam |
1.25 g IV q6h,
infused over 30 minutes |
CRE, DTR-P. aeruginosa |
|
Levofloxacin |
750 mg IV/PO q24h |
ESBL-E, AmpC-E, S. maltophilia |
|
Meropenem |
Cystitis (standard infusion): 1 g IV q8h, infused over 30 minutes All other ESBL-E or
AmpC-E infections: 1-2 g IV q8h, infused over
30 minutes All other CRE and CRAB infections: 2
g IV q8h, infused over
3 hours |
ESBL-E, AmpC-E, CRE, CRAB |
|
Meropenem- vaborbactam |
4 g IV q8h, infused over 3 hours |
CRE |
|
Minocycline |
200 mg IV/PO q12h |
CRAB, S. maltophilia |
|
Nitrofurantoin |
Cystitis: Macrocrystal/monohydrate (Macrobid®) 100 mg PO
q12h Cystitis: Oral suspension: 50 mg PO q6h |
ESBL-E
cystitis, AmpC-E cystitis |
|
Plazomicin |
Cystitis: 15 mg/kg IV x 1 dose All other infections: 15 mg/kg IV x 1 dose, subsequent doses and dosing
interval based on pharmacokinetic evaluation |
ESBL-E, AmpC-E, CRE, DTR-P. aeruginosa |
|
Polymyxin B |
Refer to international consensus guidelines on
polymyxins |
DTR-P. aeruginosa, CRAB |
|
Tigecycline |
200 mg IV x 1
dose, then 100 mg IV q12h |
CRE, CRAB, S. maltophilia |
|
Agent |
Adult Dosage (assuming
normal renal and liver function ) |
Target Organisms |
|
Tobramycin |
Cystitis: 5 mg/kg/dose IV x 1 dose All other infections: 7 mg/kg/dose IV x 1 dose; subsequent doses
and dosing interval based on pharmacokinetic
evaluation |
ESBL-E, AmpC-E, CRE, DTR-P. aeruginosa |
|
Trimethoprim- sulfamethoxazole |
Cystitis: 160
mg (trimethoprim component) IV/PO q12h Other infections: 8-12 mg/kg/day (trimethoprim component) IV/PO divided q8-12h (consider maximum dose of 960 mg trimethoprim component per day) |
ESBL-E, AmpC-E, S. maltophilia |
AmpC-E: AmpC
β-lactamase-producing Enterobacterales; CRAB:
Carbapenem-resistant Acinetobacter
baumannii; CRE: Carbapenem-resistant Enterobacterales; DTR-P.
aeruginosa: Pseudomonas
aeruginosa with difficult-to-treat resistance; E. coli: Escherichia
coli; ESBL-E: Extended-spectrum
β-lactamase-producing Enterobacterales; IV:
Intravenous; MIC: Minimum inhibitory concentration; PO: By mouth; q4h: Every 4 hours; q6h:
Every 6 hours; q8h: Every 8 hours; q12h: Every 12 hours; q24h: Every 24 hours;
S. maltophilia: Stenotrophomonas
maltophilia